Innovate Articles
AIA Supplemental Examination Nuts and Bolts: Get it in your toolbox and don’t leave home without it!
By Adriana L. Burgy; Amanda K. Murphy, Ph.D.; Sneha Nyshadham, Stacy Lewis,
Edited by Thomas L. Irving[1],[2]
Effective September 16, 2012, the America Invents Act allows a patent owner to request Supplemental Examination of a patent by the U.S. Patent and Trademark Office (“USPTO”). Supplemental Examination (“SE”) gives patent owners a proactive tool to have the USPTO consider, reconsider, or correct information that the patent owner believes is relevant to the patent. 35 U.S.C. § 257(a). Such information may include issues raised in inequitable conduct or unclean hands challenges against the patent during litigation.
Only a patent owner may request SE. 37 CFR § 1.601(a). The request may be filed at any time after patent issuance during the period of enforceability of the patent. 37 CFR § 1.601(c). After receiving a request for SE, the Director of the USPTO (“Director”) will conduct and conclude the SE within three months by issuing a certificate indicating whether the information presented in the request raises a substantial new question of patentability (“SNQ”). 35 U.S.C. § 257(a). If there is an SNQ, the patent proceeds to ex parte reexamination. If there is no SNQ, the patent remains as is, but the patent owner reaps the defensive benefits of having patentability confirmed and estopping any future unenforceability challenges based on the submitted information.
Although in effect for almost 7 years, SE is so far rarely used by patent owners. According to Finnegan’s research, based on the USPTO’s Public Patent Application Information Retrieval (“PAIR”) system, only 246 SE have been filed as of May 15, 2019, which averages to about 30 filed per year. Of the 246 requests filed for SEs, only 6 were filed so far in 2019.
Fig. 1: SE Requests Filed by Year
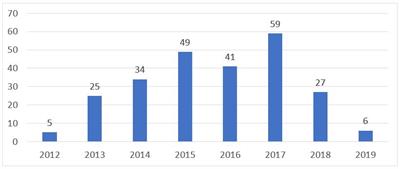
Finnegan research on PAIR, as of May 15, 2019, 246 SE requests filed
The USPTO categorizes SE requests into four broad categories in its annual reports: electrical, mechanical, chemical, and design. According to Finnegan’s research on PAIR, shown below, electrical-related requests comprise almost half of the total requests filed (110/246 (45%)). Mechanical-related requests were the second-most common with 76 (31%) requests. Chemical-related requests come in third with 57 (23%). Lastly, design requests were the least common with just 3 (1%) as of May 15, 2019.
Fig. 2: SE Requests Filed by USPTO Technology Grouping
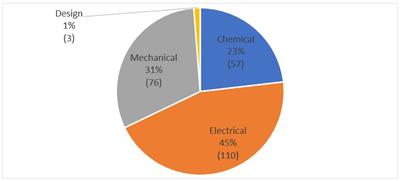
Finnegan research on PAIR, as of May 15, 2019, 246 SE requests filed.
What Materials Are Submitted with SE Requests?
The USPTO refers to the materials forming the basis of a SE proceeding as “items of information.” Items of information are “information believed to be relevant to the patent” that the patent owner wants the USPTO to consider, correct, or reconsider. 35 U.S.C. §257(a); 37 C.F.R. § 1.605(b). Note that the standard for submission is whether an item of information is “relevant,” which is not defined in the statute but is broader than whether information is “material” under 37 C.F.R. §1.56.[3] Additionally, items of information are required to be in writing. 37 C.F.R. § 1.605(c). Therefore, to be considered, any audio or video recording must be submitted in the form of a written transcript. Id.
Each request is limited to no more than twelve items of information, but multiple requests for SE of the same patent at any time may be submitted. 37 C.F.R. § 1.605(a). This means the patent owner can submit several requests of up to 12 items each if he or she would like to have more than 12 items considered by the USPTO.
Of course, SE is not free. Presently, the fees associated with a SE are $4,400 for processing a request for SE, and $12,100 if ex parte reexamination is ordered as a result of a SE proceeding. In addition, there are fees for documents over certain page limits. 37 C.F.R. §1.20(k); 37 C.F.R. §1.610(a). Small-entity and micro-entity discounts, however, are applied.
Since inception, the types of items of information submitted for SE have not been limited to just patents and patent applications. Instead, approximately 1/3 of the requests include printed publications, declarations, and webpages. Those materials also included descriptions of physical items, descriptions of sales/public uses (not in a declaration), and citations to case law and the MPEP discussing Alice Corp. v. CLS Bank Int’l, 573 U.S. 208 (2014) (“Alice”) and subject matter eligibility. The remaining 2/3 of requests include U.S. and foreign patents or patent applications. Such requests appear to be filed to ensure that the USPTO considers patents and patent applications that were cited in foreign counterpart prosecution or during prosecution of related U.S. patents/patent applications. This “patents/applications” category of information, as shown below in the diagram, also included related search reports and prosecution history documents.
Fig. 3: What Kinds of Materials Are Submitted with SE Requests?
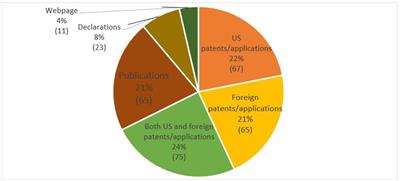
Finnegan research on PAIR, as of May 15, 2019, 246 SE requests filed. May be more than one type of material submitted per request. “Patents/applications” includes related search reports and prosecution history documents. Other materials include MPEP, case law discussing Alice and subject matter eligibility, physical items, and descriptions of on sale/public use not in a declaration.
Substantial New Question of Patentability and Ex Parte Reexamination
According to Finnegan’s research on PAIR, a SNQ was not found for 69 (28%) of the 246 SEs. Excluding one pending and one terminated case, this shows that a SNQ was found in the vast majority of cases (175 (72%)).
Fig. 4: Substantial New Question of Patentability (“SNQ”) Found?
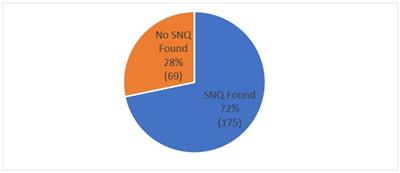
Finnegan research on PAIR, as of May 15, 2019, 246 SE requests filed. Not included in chart: 1 case terminated and 1 pending.
If a SNQ is found by one or more items of information in the request, the Director, as noted above, will order ex parte reexamination of the patent. 35 U.S.C. § 257(b). During reexamination, the Director will address each SNQ identified during the SE. 35 U.S.C. § 257. That means ex parte reexamination instituted as a result of SE is, as suggested above, not limited to patents and printed publications, in contrast to ex parte reexamination instituted under 35 U.S.C. §§ 301 and 302.
After an ex parte reexamination proceeding is complete, an ex parte reexamination certificate is published, indicating that the reexamined patent claims are either cancelled, amended, newly added, or left the same. According to Finnegan’s research on PAIR, 19% (52) of the 148 reexamination certificates that were issued had at least one original claim confirmed. Another 19% (52) of the reexamination certificates that were issued contained at least one new claim. Furthermore, 32% (88) of the reexamination certificates that were issued had at least one claim amended and 30% (82) had at least one claim cancelled. This showed that over 3/4 of the time, at least one original claim of the patent was not confirmed as of May 15, 2019.
Fig. 5: Breakdown When Reexam Certificate Issued
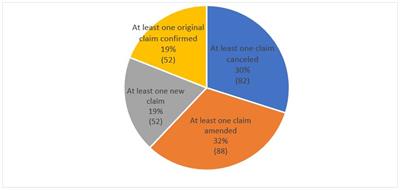
Finnegan research on PAIR, as of May 15, 2019, 148 reexam certificates issued.
Although 175 SE requests found a SNQ, only 148 of those requests received a reexamination certificate. Out of the remaining 27 requests, 1 has a pending appeal to the Court of Appeals for the Federal Circuit, 1 has a pending appeal to the Patent Trial and Appeal Board, 1 had a co-pending inter partes review (“IPR”) in which all of the patent’s claims were found unpatentable and so the SE request was dismissed, 4 have a notice of intent to issue a reexamination certificate, and 20 are still pending for reexamination outcomes. For the 148 requests in which a reexamination certificate was issued, the average pendency from finding a SNQ until issuance of the reexamination certificate was 433 days (14+ months).
Conclusion
In summary, the records show that SE can be beneficial to patent owners. The proceeding must be completed within three months from filing the request, making it an extremely speedy process. If no SNQ is raised by any of the items of information in the request, a SE certificate is published, and the patent remains as granted with all of the defensive benefits that are derived from having gone through SE. The short time period for determining whether there is a SNQ may benefit a patent owner who may foresee patent litigation on the horizon. Furthermore, patent owners can submit a wide variety of documents for consideration and are not limited to patents and printed publications, as in other post-grant proceedings. And since the standard for submission is a relatively low bar of relevance, no great amount of time must be spent determining materiality of the items of information. SE’s ability to cover a wide range of documents and related issues in a short amount of time may benefit patent owners and their patents. Given these benefits, we would not be surprised if use of SE by patent owners increases with time.
[1] DISCLAIMER: These materials have been prepared solely for educational and entertainment purposes to contribute to the understanding of U.S. intellectual property law. These materials reflect only the personal views of the authors and are not individualized legal advice. It is understood that each case is fact specific, and that the appropriate solution in any case will vary. Therefore, these materials may or may not be relevant to any particular situation. Thus, the authors and Finnegan, Henderson, Farabow, Garrett & Dunner, LLP (including Finnegan Europe LLP, and Fei Han Foreign Legal Affairs Law Firm) cannot be bound either philosophically or as representatives of their various present and future clients to the comments expressed in these materials. The presentation of these materials does not establish any form of attorney-client relationship with these authors. While every attempt was made to ensure that these materials are accurate, errors or omissions may be contained therein, for which any liability is disclaimed.
[2] Tom Irving is a Finnegan partner. Amanda Murphy is a Finnegan partner. Adriana Burgy is a Finnegan partner. Sneha Nyshadham is a Finnegan summer associate. Stacy Lewis is a Finnegan law clerk.
[3] 37 C.F.R. 1.56(b) defines “material” as follows:
Under this section, information is material to patentability when it is not cumulative to information already of record or being made of record in the application, and
(1) It establishes, by itself or in combination with other information, a prima facie case of unpatentability of a claim; or
(2) It refutes, or is inconsistent with, a position the applicant takes in:
(i) Opposing an argument of unpatentability relied on by the Office, or
(ii) Asserting an argument of patentability.
A prima facie case of unpatentability is established when the information compels a conclusion that a claim is unpatentable under the preponderance of evidence, burden-of-proof standard, giving each term in the claim its broadest reasonable construction consistent with the specification, and before any consideration is given to evidence which may be submitted in an attempt to establish a contrary conclusion of patentability.
and management, and litigation in the chemical, pharmaceutical, and biotechnology arts.
Adriana brings a unique perspective by blending her legal, technical, and industry
experience.
Amanda Murphy, Ph.D., focuses her practice on strategic client counseling, portfolio
management, and patent prosecution for a range of clients, including small startup
companies, research foundations, and large biotechnology and pharmaceutical companies.
Tom Irving has more than 40 years of experience in the field of intellectual property law.
His practice includes America Invents Act (AIA) post-grant proceedings, due diligence,
counseling, patent prosecution, and reissue and reexamination. He counsels clients on a
wide range of mainly pharmaceutical matters, including pre-litigation, Orange Book
listings of patents covering FDA-approved drugs, infringement issues, enforceability and
validity analysis.
